Which electron configurations of neutral atoms represent excited states? Delving into this intriguing realm, we embark on a journey to unravel the intricate relationship between electron configurations and the energy levels of atoms. This exploration unveils the conditions under which atoms transition to excited states, the spectroscopic methods employed to determine their electron configurations, and the practical applications that harness their unique properties.
1. Define Excited State of Atoms: Which Electron Configurations Of Neutral Atoms Represent Excited States

An excited state of an atom is an unstable state in which one or more of its electrons occupies an energy level higher than the ground state. In the ground state, all electrons are in their lowest energy levels. When an atom absorbs energy, such as from light or heat, an electron can be excited to a higher energy level, creating an excited state.
2. Electron Configurations of Excited States
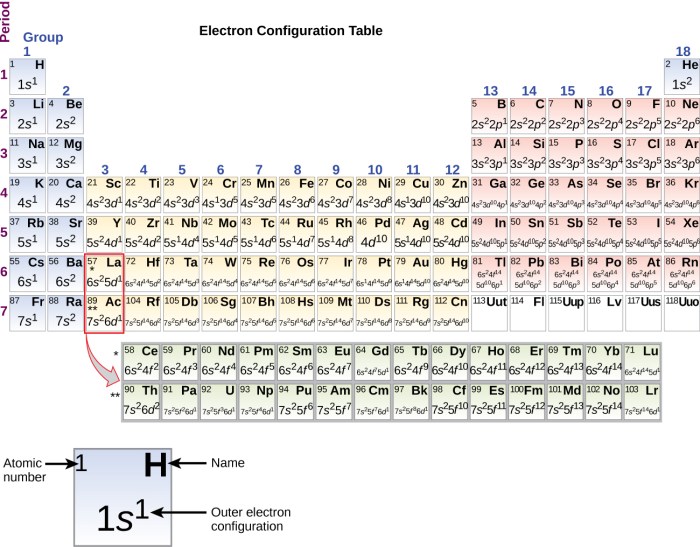
The electron configuration of an atom in an excited state differs from that of the ground state. In an excited state, one or more electrons occupy higher energy orbitals. These changes in electron configuration can be represented using spectroscopic notation, which indicates the energy level, subshell, and spin of each electron.
Examples of Electron Configurations Representing Excited States
- Hydrogen (H): 1s 2(ground state) → 1s 12s 1(excited state)
- Helium (He): 1s 2(ground state) → 1s 12p 1(excited state)
- Lithium (Li): 1s 22s 1(ground state) → 1s 22p 1(excited state)
3. Methods for Determining Excited State Electron Configurations
Spectroscopic methods are used to determine the electron configurations of excited states. These methods include:
Atomic Emission Spectroscopy
When an excited atom returns to the ground state, it releases energy in the form of light. The wavelength of the emitted light corresponds to the energy difference between the excited state and the ground state. By analyzing the emitted light, the electron configuration of the excited state can be determined.
Atomic Absorption Spectroscopy
This technique measures the amount of light absorbed by an atom when it is excited to a higher energy level. The wavelength of the absorbed light corresponds to the energy difference between the ground state and the excited state. By analyzing the absorbed light, the electron configuration of the excited state can be determined.
Quantum Mechanics
Quantum mechanics provides a theoretical framework for calculating the electron configurations of excited states. By solving the Schrödinger equation for the atom, the energy levels and electron configurations of all possible excited states can be determined.
4. Applications of Excited State Electron Configurations
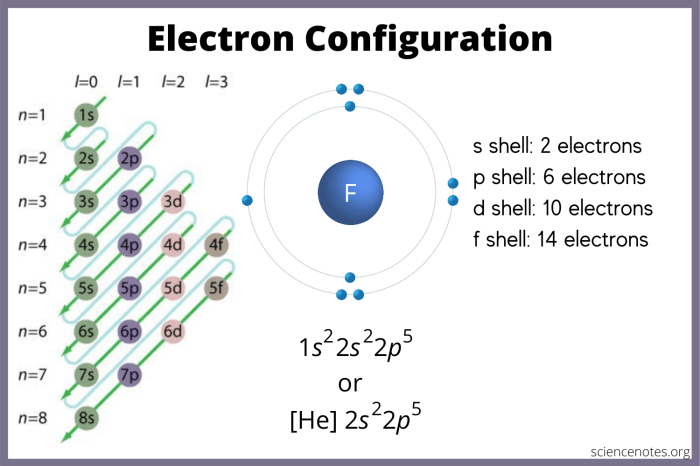
Excited state electron configurations play a crucial role in various fields, including:
Chemical Reactions
Excited states can participate in chemical reactions, leading to the formation of new molecules and compounds. For example, in photosynthesis, chlorophyll absorbs light and enters an excited state, which initiates the electron transfer reactions necessary for the conversion of carbon dioxide into glucose.
Astrophysics
Excited states of atoms are responsible for the emission of light in stars and other celestial objects. By analyzing the light emitted from stars, astronomers can determine the elemental composition and temperature of these objects.
Plasma Physics, Which electron configurations of neutral atoms represent excited states
Excited states are present in plasmas, which are ionized gases found in various applications, such as fusion reactors and plasma displays. The electron configurations of excited states in plasmas influence the plasma’s behavior and properties.
Helpful Answers
What is an excited state of an atom?
An excited state occurs when an atom absorbs energy, causing its electrons to occupy higher energy levels than their ground state.
How do changes in electron configurations lead to excited states?
When electrons transition to higher energy levels, the atom’s electron configuration changes, resulting in an excited state.
What methods are used to determine excited state electron configurations?
Spectroscopic techniques, such as atomic emission and absorption spectroscopy, are commonly used to analyze the wavelengths of light emitted or absorbed by atoms, providing insights into their excited state electron configurations.