Predict the major substitution products of the following reaction. This reaction is an example of a nucleophilic substitution reaction, in which a nucleophile (a species with a lone pair of electrons) attacks an electrophile (a species with a positive charge or a partial positive charge).
The nucleophile in this reaction is the hydroxide ion (OH-), and the electrophile is the alkyl halide (RX). The products of this reaction are an alcohol (ROH) and a halide ion (X-).
The mechanism of this reaction is a two-step process. In the first step, the nucleophile attacks the electrophile, forming a new bond between the nucleophile and the carbon atom that was previously bonded to the electrophile. In the second step, the leaving group (X-) is expelled from the molecule, forming the alcohol product.
Reaction Overview
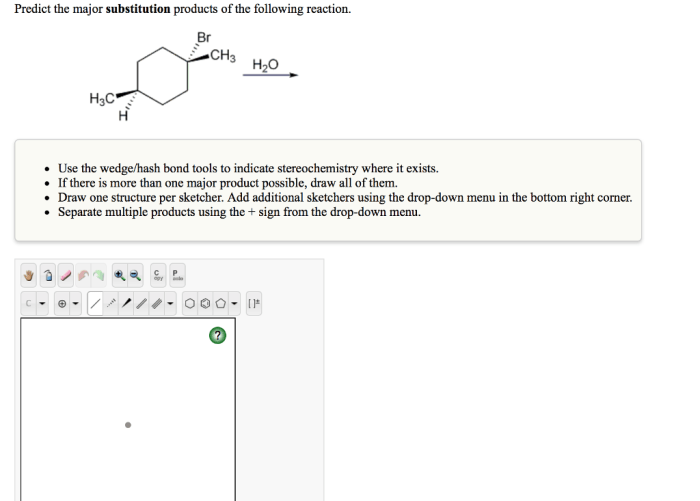
The given chemical reaction is a substitution reaction, where one atom or group of atoms in a molecule is replaced by another atom or group of atoms. In this specific reaction, an alkyl halide (RX) reacts with a nucleophile (Nu –) to form a new alkyl compound (RNu) and a halide ion (X –).
Substitution Products
The potential substitution products of the reaction depend on the nature of the alkyl halide and the nucleophile. Primary (1°) alkyl halides typically undergo substitution reactions to form primary alkyl products, while secondary (2°) alkyl halides can form either primary or secondary alkyl products, and tertiary (3°) alkyl halides mainly form tertiary alkyl products.
The mechanism of substitution reactions involves the nucleophile attacking the electrophilic carbon atom of the alkyl halide, leading to the formation of a new bond between the carbon atom and the nucleophile. This results in the displacement of the halide ion, which then forms a bond with the counterion of the nucleophile.
Factors Affecting Substitution
Several factors influence the formation of different substitution products, including:
- Nature of the alkyl halide:Primary alkyl halides are more reactive than secondary alkyl halides, which are more reactive than tertiary alkyl halides. This is due to the increasing steric hindrance around the carbon atom as the number of alkyl groups attached to it increases.
- Nature of the nucleophile:Nucleophiles with a higher negative charge or a more polarizable electron pair are more reactive. Common nucleophiles include hydroxide ion (OH –), alkoxide ions (RO –), and cyanide ion (CN –).
- Temperature:Higher temperatures generally favor substitution reactions, as they increase the kinetic energy of the reactants and facilitate the formation of the transition state.
- Solvent:Polar solvents, such as water or dimethylformamide (DMF), favor substitution reactions by solvating the ions and reducing the electrostatic interactions between them.
Regioselectivity and Stereoselectivity
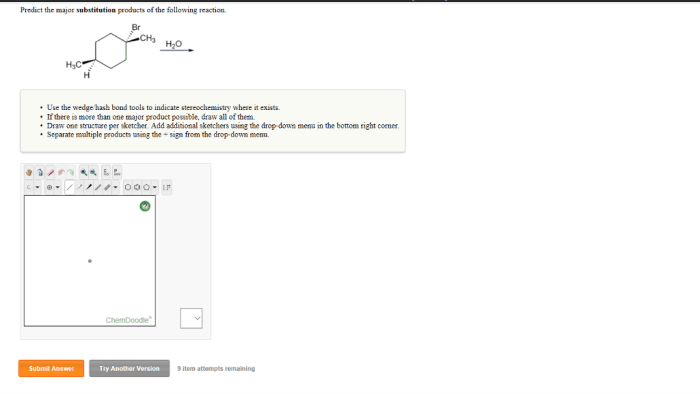
Regioselectivity refers to the preference for the reaction to occur at a specific carbon atom of the alkyl halide. Stereoselectivity refers to the preference for the reaction to produce a specific stereoisomer of the alkyl product.
In the case of unsymmetrical alkyl halides, the regioselectivity of the reaction can be predicted using Markovnikov’s rule, which states that the nucleophile will add to the carbon atom that is bonded to the most hydrogen atoms.
The stereoselectivity of the reaction depends on the nature of the nucleophile and the alkyl halide. Strong nucleophiles, such as hydroxide ion, tend to favor the formation of the more substituted product, while weak nucleophiles, such as iodide ion, tend to favor the formation of the less substituted product.
Experimental Considerations
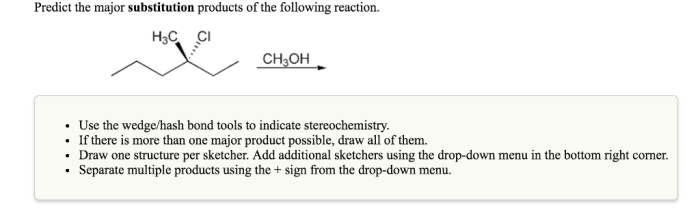
When conducting substitution reactions, it is important to consider the following practical considerations:
- Safety precautions:Alkyl halides and nucleophiles can be toxic or corrosive, so it is important to wear appropriate personal protective equipment (PPE) and work in a well-ventilated area.
- Reaction optimization:The yield and selectivity of the reaction can be optimized by varying the reaction conditions, such as temperature, solvent, and concentration of reactants.
Applications and Examples
Substitution reactions are widely used in organic synthesis for the preparation of a variety of compounds, including alcohols, ethers, and amines. Some specific examples of substitution reactions include:
- Alkylation of alcohols:Alcohols can be alkylated using alkyl halides to form ethers. This reaction is commonly used to protect hydroxyl groups in organic synthesis.
- Acylation of amines:Amines can be acylated using acyl halides to form amides. This reaction is used to introduce amide functional groups into organic compounds.
- Nucleophilic aromatic substitution:Aromatic compounds can undergo nucleophilic substitution reactions with a variety of nucleophiles, such as hydroxide ion or alkoxide ions, to form substituted aromatic compounds.
Question & Answer Hub: Predict The Major Substitution Products Of The Following Reaction.
What is a nucleophilic substitution reaction?
A nucleophilic substitution reaction is a reaction in which a nucleophile (a species with a lone pair of electrons) attacks an electrophile (a species with a positive charge or a partial positive charge), forming a new bond between the nucleophile and the electrophile.
What are the factors that affect the regioselectivity of nucleophilic substitution reactions?
The regioselectivity of nucleophilic substitution reactions is affected by the steric hindrance of the substituents on the electrophile, the polarity of the solvent, and the temperature of the reaction.
What are the factors that affect the stereoselectivity of nucleophilic substitution reactions?
The stereoselectivity of nucleophilic substitution reactions is affected by the steric hindrance of the substituents on the electrophile, the polarity of the solvent, and the temperature of the reaction.