For each pair of biomolecules identify the type of reaction, a concept that lies at the heart of biochemistry. This exploration unveils the intricate relationships between biomolecules, revealing the mechanisms that govern their interactions and drive the symphony of life.
Biomolecules, the building blocks of life, engage in a mesmerizing dance of chemical reactions, each type orchestrated by specific rules and forces. From the hydrolysis that breaks down complex molecules to the dehydration synthesis that forges new bonds, these reactions shape the very fabric of biological systems.
Biochemical Reactions
Biochemical reactions are chemical reactions that occur within living organisms and are essential for life. They facilitate the transformation of molecules and energy, enabling cells to function and carry out their biological processes.
Biochemical reactions can be classified into four main types: hydrolysis, dehydration synthesis, oxidation-reduction, and energy transfer.
Hydrolysis
Hydrolysis reactions involve the breakdown of a molecule by the addition of water. This process is commonly used to break down complex molecules into simpler ones, such as the digestion of proteins into amino acids.
Dehydration Synthesis
Dehydration synthesis reactions are the reverse of hydrolysis, where two molecules are joined together with the removal of a water molecule. This process is used to synthesize complex molecules from simpler ones, such as the formation of proteins from amino acids.
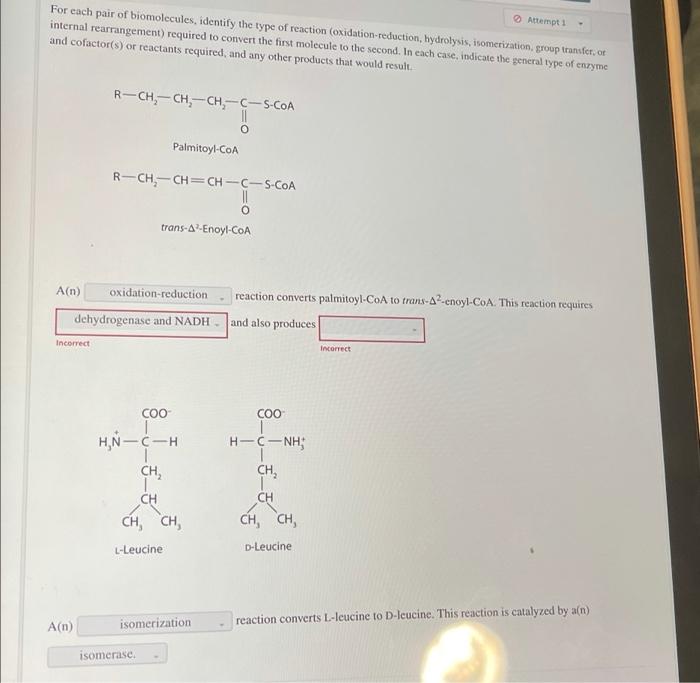
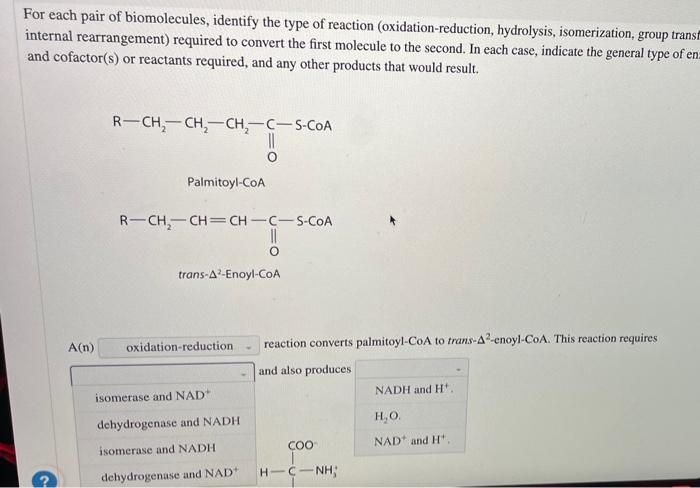
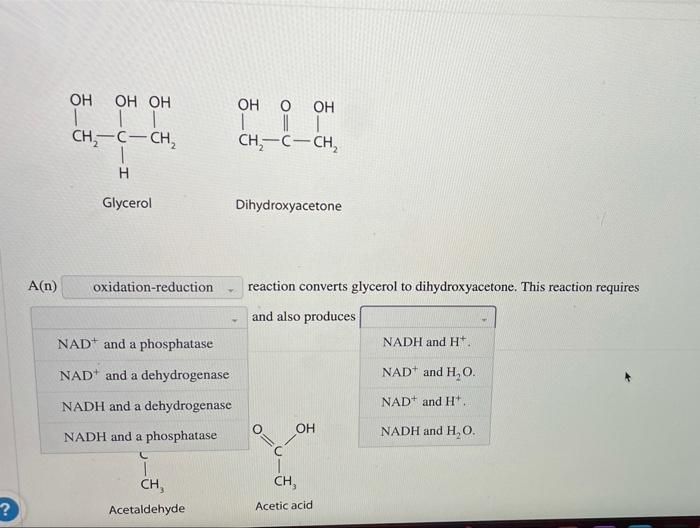
Oxidation-Reduction
Oxidation-reduction reactions involve the transfer of electrons between molecules. Oxidation refers to the loss of electrons, while reduction refers to the gain of electrons. These reactions are crucial for energy production and metabolism.
Energy Transfer, For each pair of biomolecules identify the type of reaction
Energy transfer reactions involve the transfer of energy from one molecule to another. This process is often coupled with other biochemical reactions to provide the necessary energy for cellular activities.
Biomolecules and their Interactions
Biomolecules are the building blocks of living organisms and perform a wide range of functions. They are classified into four main groups: carbohydrates, proteins, lipids, and nucleic acids.
Carbohydrates
Carbohydrates are composed of carbon, hydrogen, and oxygen atoms and serve as the primary source of energy for cells. They include sugars, starches, and cellulose.
Proteins
Proteins are composed of amino acids and play a crucial role in cell structure, function, and regulation. They include enzymes, hormones, and antibodies.
Lipids
Lipids are a diverse group of molecules that include fats, oils, and waxes. They serve as energy reserves, provide insulation, and form cell membranes.
Nucleic Acids
Nucleic acids are composed of nucleotides and carry genetic information. They include DNA and RNA, which are essential for protein synthesis and cellular reproduction.
Interactions between Biomolecules
Biomolecules interact with each other through various types of bonds and forces, including:
- Covalent bonds: Strong chemical bonds that form between atoms, holding molecules together.
- Hydrogen bonds: Weaker bonds that form between electronegative atoms and hydrogen atoms, contributing to molecular structure and interactions.
- Hydrophobic interactions: Non-polar molecules or regions of molecules that interact with each other in aqueous environments.
- Ionic bonds: Electrostatic attractions between charged atoms or molecules.
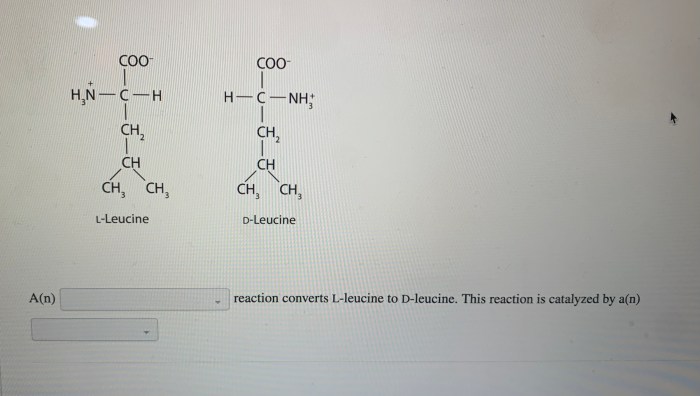
Identifying Reaction Types for Biomolecule Pairs
| Biomolecule 1 | Biomolecule 2 | Reaction Type | Example |
|---|---|---|---|
| Glucose | Water | Hydrolysis | Glucose + Water → Glucose-1-phosphate |
| Amino acids | Dehydration synthesis | Amino acids + Water → Peptide bond | |
| Alcohol | Oxygen | Oxidation-reduction | Alcohol + Oxygen → Aldehyde + Water |
| ATP | ADP | Energy transfer | ATP + ADP → ADP + Energy |
Significance of Reaction Type Identification
Identifying the type of reaction between biomolecules is crucial for understanding biological processes. It allows researchers to:
- Predict the outcome of biochemical pathways.
- Design new therapeutic strategies.
- Develop drugs that target specific biochemical reactions.
- Understand the mechanisms of disease.
FAQ Explained: For Each Pair Of Biomolecules Identify The Type Of Reaction
What is the significance of identifying reaction types for biomolecule pairs?
Understanding reaction types provides insights into biological processes, enabling researchers to predict outcomes and design therapeutic strategies.
How do the chemical structures of biomolecules influence reaction types?
Functional groups and molecular architecture play crucial roles in determining the type of reaction that occurs between biomolecules.